
Client Case Study
This case describes a female patient in her early 40s, a single mother, diagnosed with Stage IIIB colon cancer. In the two or more years preceding diagnosis, the patient repeatedly presented to her primary care provider with complaints of progressive fatigue, declining energy, insomnia, and a significant reduction in physical fitness. These symptoms were attributed to depression or anxiety, and antidepressant therapy was recommended; the patient declined pharmacologic treatment.
The cancer diagnosis was ultimately precipitated when the patient was contacted to participate in a 12-year follow-up study conducted by the Barbara Davis Center, during which concerning findings prompted urgent referral back to her primary care provider for further evaluation.
Following partial colectomy with unclear cancer margins extending into the mesentery, adjuvant chemotherapy was strongly recommended but declined by the patient. This case outlines the subsequent clinical course, biochemical markers, and long-term outcomes observed over nearly a decade of follow-up.
Findings of Radical Remission
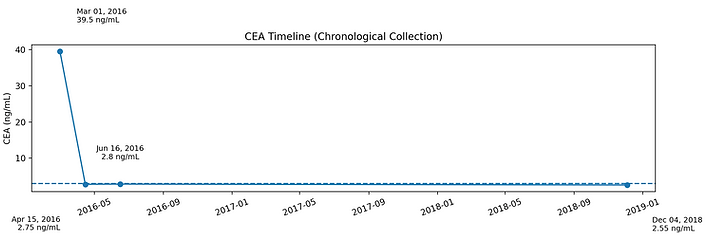
This CEA trend is presented in the context of Stage IIIB colon cancer, including partial colectomy with unclear surgical margins extending into the mesentery. Adjuvant chemotherapy was declined.
Initial postoperative testing demonstrated a markedly elevated CEA (39.5 ng/mL on Mar 01, 2016), followed by rapid normalization into the reference range by Apr 15, 2016, with sustained normalization on subsequent measurements (Jun 16, 2016 and Dec 04, 2018).
This trajectory reflects a significant and sustained biochemical response following diagnosis, with no subsequent CEA elevation over the documented follow-up period. This pattern is clinically atypical, given the presence of unclear margins and the strong recommendations for adjuvant chemotherapy typically made to reduce recurrence risk and improve survival in Stage IIIB disease.
The patient has experienced no further cancer-related complications as of January 2026.
Carcinoembryonic Antigen (CEA)
Composite Laboratory Results (Chronological Order)
Test Summary Table
Collection DateCollection TimeCEA Result (ng/mL)Reference Range (ng/mL)Interpretation
Mar 01, 20162:40 PM39.50.00 – 3.00High
Apr 15, 201612:25 PM2.750.00 – 3.00Within Range
Jun 16, 20169:55 AM2.80.00 – 3.00Within Range
Dec 04, 20183:46 PM2.550.00 – 3.00Within Range
Individual Result Details
Mar 01, 2016
-
Test: Carcinoembryonic Antigen (CEA)
-
Result: 39.5 ng/mL (High)
-
Reference Range: 0.00 – 3.00 ng/mL
-
Ordering Provider: Charles Jones, MD
-
Result Status: Final
-
Resulting Lab: FTH Lab Legacy, 4747 Arapahoe Ave., Boulder, CO 80303
-
Lab Director: Kevin W. Hanley
Apr 15, 2016
-
Test: Carcinoembryonic Antigen (CEA)
-
Result: 2.75 ng/mL
-
Reference Range: 0.00 – 3.00 ng/mL
-
Interpretation: Within normal limits
Jun 16, 2016
-
Test: Carcinoembryonic Antigen (CEA)
-
Result: 2.8 ng/mL
-
Reference Range: 0.00 – 3.00 ng/mL
-
Interpretation: Within normal limits
Dec 04, 2018
-
Test: Carcinoembryonic Antigen (CEA)
-
Result: 2.55 ng/mL
-
Reference Range: 0.00 – 3.00 ng/mL
-
Interpretation: Within normal limits
-
Additional Note: EDTA plasma shows approximately −13% bias compared to serum
Clinical Interpretation Summary
This CEA trend is presented in the context of Stage IIIB colon cancer, status post partial colectomy with unclear margins extending into the mesentery. Adjuvant chemotherapy was declined. Initial postoperative testing demonstrated a markedly elevated CEA (39.5 ng/mL on Mar 01, 2016), followed by rapid normalization into the reference range by Apr 15, 2016, with sustained normalization on subsequent measurements (Jun 16, 2016 and Dec 04, 2018). This pattern reflects a significant biochemical response following surgical intervention, with no subsequent CEA elevation over the documented follow‑up period.
Clinical Notes
-
Results are presented in chronological order based on collection date.
-
Reference ranges are laboratory-reported at the time of testing.
-
This composite is intended for clinical review and historical comparison.



.png)

